Debate regarding the morality of CRISPR/Cas9 therapy has been sparked by the revelation nearly two years ago that a researcher in China had used the technology to edit the germline of twins. Immediately following, the director of the National Institutes of Health (NIH), Francis Collins, condemned the germline editing as a "scientific misadventure" and even "disturbing," stating clearly that the NIH "does not support the use of gene-editing technologies in human embryos".1
An article in Health Care Ethics USA written by Paul Scherz of the Catholic University of America highlights the legitimate uses and major problems of CRISPR.2 Briefly, the major current benefits of CRISPR are its use in somatic cell lines as a research tool to streamline procedural workflows and as a possible therapeutic agent for somatic cells. The major ethical problems with CRISPR relate to germline editing. Scherz notes that these problems include technical challenges such as mosaicism, off-target effects, imprecision, and general lack of knowledge of the intended effects, which increase the risk of unintended harms. Additionally concerning from a Catholic perspective, the process utilizes in vitro fertilization (IVF) and other embryonic manipulation which disrupts the unitive component of the marital act and harms the dignity of persons at the earliest stage of development. Finally, for some, the intention of gene editing may be aimed at trait enhancement instead of therapeutic treatment of disease, which may lead to a type of eugenics.3 Our letter is intended to resolve two of these ethical concerns, with a highlight on a scientific advance which specifically addresses IVF and embryonic manipulation.
First, scientific problems such as mosaicism, off-target effects, and imprecision have long been known and are all issues that the scientific community is well aware of. Tenacious work has led to many improvements in CRISPR over the years (for reviews, see references 4 and 5) and even the first clinical trials of CRISPR in humans utilizing somatic gene editing for beta-thalassemia (NCT03655678) and an inherited form of blindness (NCT03872479).
History has demonstrated that even seemingly impenetrable technical difficulties in genetic manipulation have been resolved over years of intense work and incremental improvements, such as the first viral gene therapy for blindness being FDA approved in late 2017.6 David Sourdive, vice president of Cellectis, a European CAR-T cell development company has stated, "We think CRISPR will get there because there are so many people working on it, improving it, that it will happen — it's just a matter of time … but it's not there yet."7 This lends to an optimistic view that, technologically, CRISPR will eventually have minimal off-target effects, minimal imprecision, and will be applied to a disease state that would be curative. If not CRISPR, other technologies are competing for the same prize of a therapeutically relevant genome editing, most notably TALENs and zinc-finger nucleases.8 As a result, the unintentional harms associated with the current technical limitations of genetic manipulation should not be presumed to serve as a sustainable ethical bulwark.
Secondly, Catholic ethicists note that embryonic manipulation in vitro would preclude moral use because it disrupts the unitive component of the marital act and frequently results in embryo loss. The Congregation for the Doctrine of the Faith (CDF) states in Dignitas Personae that a major problem with this type of therapy is that "gene therapy on an embryo, … only takes place in the context of in vitro fertilization and thus runs up against all the ethical objections to such procedures."9 Because IVF is not ethically problematic in the eyes of many scientists one might presume that this technical problem is unlikely to be addressed. However, the scientific community may have serendipitously discovered a technique to perform gene therapy on an embryo that does not disrupt the unitive nature of the marital act central to Catholic moral teaching.
This is called the GONAD technique. GONAD (Genome‐editing via Oviductal Nucleic Acids Delivery) utilizes CRISPR/Cas9 technology to genetically modify a zygote at the one- or two-cell stage without removing the zygote from the fallopian tube10 (Figure 1). Briefly, the zygote is conceived through natural intercourse. At a predetermined time after conception, an incision is made, and the fallopian tube exposed. The gene editing agent along with a dye is injected into the fallopian tube, and then the tube is electroporated (electrically shocked) to facilitate the transfer of the agent into the zygote. The technique grew from a discovery in 2012 that plasmid DNA could be transferred to a mouse embryo in this manner.11 In 2015, the first use of Cas9 to disrupt a gene with the GONAD technique was reported.12 Finally, in 2018 improvements in both the timing of delivery and gene editing agent resulted in a 97% success rate for introducing insertions or deletions in the mouse offspring genome. Other proof-of-concept gene editing technologies were demonstrated to be effective using the same technique.13, 14

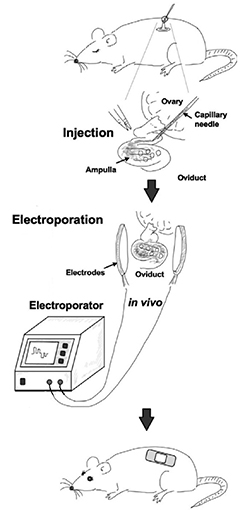 Figure 1. Overview of GONAD procedure. Mice are mated and about 16 hours post-mating the pregnant female is put under general anesthesia. The fallopian tube is exposed, the gene-editing solution is injected, and the fallopian tube is electroporated to facilitate gene transfer. The incision is closed, and the mouse undergoes a normal pregnancy. Figure adapted from reference 10. Figure permission is pending.
Figure 1. Overview of GONAD procedure. Mice are mated and about 16 hours post-mating the pregnant female is put under general anesthesia. The fallopian tube is exposed, the gene-editing solution is injected, and the fallopian tube is electroporated to facilitate gene transfer. The incision is closed, and the mouse undergoes a normal pregnancy. Figure adapted from reference 10. Figure permission is pending.
While the primary application of this technique is to create genetically engineered mouse models in a cheaper and faster manner (as opposed to microinjection of zygotes)15, the GONAD technique has been generalized to use in rats16, 17 and is predicted to be able to be used in mammals such as cow and pig.10
Assuming that the future sees the development of a safe, reliable, and highly efficient genome editing technology, and provided the technology is used in a situation that is strictly therapeutic, future iterations of the GONAD technique could possibly be used in humans for moral, therapeutic genome editing of embryos.
The reasons why this technique may be morally licit from a Catholic perspective are twofold. First, it does not disrupt the marital act, meaning that the unitive and procreative ends of sexual intercourse would remain intact. The GONAD technique solves this issue because it relies on timing of the act of copulation so that the zygote is in the one- or two-cell stage when transfected with the gene editing agent while still within the fallopian tube.
Second, it does not denigrate the value of the embryo. The embryo is truly a human being that has all the rights of a person, including the right to life and, unique to the embryo, the right "to be conceived and to be born within marriage and from marriage"18 e.g., the right to begin life within its mother. The GONAD technique also solves this issue because the embryo is conceived within the mother after the marital act and undergoes a normal gestation and birth.
It has to be admitted that the GONAD technique is far from being used in humans. In addition to the overcoming the current hurdles of mosaicism, off-target effects, etc., with gene editing technologies, the GONAD technique would have to be developed further such that those gene editing technologies don't represent a significant risk to the safety of the embryo or mother. Nevertheless, this may be the first scientifically sound technique for embryonic gene therapy in mammals that does not violate the basic rights of all humans, and therefore, it is worth watching its development for the future.
Finally, the advances discussed in this letter do not resolve other challenging ethical questions such as distinguishing between therapeutic uses and enhancement, guarding against eugenic intentions, or protecting future generations.
While Scherz's article rightly reflects the current state of CRISPR, the article does not adequately anticipate the likely success the scientific community will have in addressing technical challenges. As they are addressed, it should not be presumed that the technical challenges will serve as a bulwark against increased adaptation of germline editing. Moreover, in vitro fertilization represents a considerable obstacle in the moral use of CRISPR in embryos. Advances that follow from the GONAD technique may circumvent the need for in vitro embryonic manipulation, paving the way for morally permissible use of gene editing technologies.
Michael Redinger, MD, MA
Co-Chief, Program in Medical Ethics, Humanities, and Law
Assistant Professor, Program in Medical Ethics, Humanities, and Law
Assistant Professor, Department of Psychiatry
Homer Stryker M.D. School of Medicine
Western Michigan University
Kalamazoo, Michigan
[email protected]
Jacob Poliskey, Ph.D.
Class of 2022
Homer Stryker M.D. School of Medicine
Western Michigan University
Kalamazoo, Michigan
[email protected]
ENDNOTES
- Collins FS. Statement on Claim of First Gene-Edited Babies by Chinese Researcher. National Institutes of Health. 2018.
- Scherz P. Health Care Ethics USA, The Rapidly Evolving Debate Over CRISPR. 2019:24-29.
- Ibid.
- Li J, Hong S, Chen W, Zuo E, Yang H. Advances in detecting and reducing off-target effects generated by CRISPR-mediated genome editing. J Genet Genomics. 2020. doi:10.1016/j.jgg.2019.11.002
- Pickar-Oliver A, Gersbach CA. The next generation of CRISPR– – Cas technologies and applications. Nat Rev Mol Cell Biol. 2019. doi:10.1038/s41580-019-0131-5
- FDA. Press Announcements FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. FDA Press Release. 2017.
- The Most Important Battle in Gene Editing: CRISPR versus TALEN.
- Gaj T, Gersbach CA, Barbas CF. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013. doi:10.1016/j.tibtech.2013.04.004
- Congregation for the Doctrine of the Faith. Instruction Dignitas Personae on Certain Bioethical Questions. 2008.
- Ohtsuka M, Sato M. i-GONAD: A method for generating genome-edited animals without ex vivo handling of embryos. Dev Growth Differ. 2019. doi:10.1111/dgd.12620
- Sato M, Akasaka E, Saitoh I, Ohtsuka M, Watanabe S. In vivo gene transfer in mouse preimplantation embryos after intraoviductal injection of plasmid DNA and subsequent in vivo electroporation. Syst Biol Reprod Med. 2012. doi:10.3109/19396368.2012.688088
- Takahashi G, Gurumurthy CB, Wada K, Miura H, Sato M, Ohtsuka M. GONAD: Genome-editing via Oviductal Nucleic Acids Delivery system: A novel microinjection independent genome engineering method in mice. Sci Rep. 2015. doi:10.1038/srep11406
- Ohtsuka M, Sato M, Miura H, et al. I-GONAD: A robust method for in situ germline genome engineering using CRISPR nucleases. Genome Biol. 2018. doi:10.1186/s13059-018-1400-x
- Sato M, Takabayashi S, Akasaka E, Nakamura S. Recent Advances and Future Perspectives of In Vivo Targeted Delivery of Genome-Editing Reagents to Germ cells, Embryos, and Fetuses in Mice. Cells. 2020. doi:10.3390/cells9040799
- Gurumurthy CB, Sato M, Nakamura A, et al. Creation of CRISPR-based germline-genome-engineered mice without ex vivo handling of zygotes by i-GONAD. Nat Protoc. 2019. doi:10.1038/s41596-019-0187-x
- Kobayashi T, Namba M, Koyano T, et al. Successful production of genome-edited rats by the rGONAD method. BMC Biotechnol. 2018. doi:10.1186/s12896-018-0430-5
- Takabayashi S, Aoshima T, Kabashima K, Aoto K, Ohtsuka M, Sato M. i-GONAD (improved genome-editing via oviductal nucleic acids delivery), a convenient in vivo tool to produce genome-edited rats. Sci Rep. 2018. doi:10.1038/s41598-018-30137-x
- Congregation for the Doctrine of the Faith. Instruction on Respect for Human Life in its Origin and on the Dignity of Procreation. 1987.