By RENEE STOVSKY
ST. LOUIS — Rosie Williams, 43, works at SSM Health Cardinal Glennon Children's Hospital here as a patient coordinator in the division of endocrinology/diabetes.
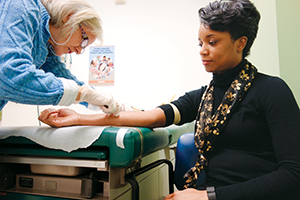
Rosie Williams, right, is one of 30 volunteers in a Zika vaccine study being conducted by Saint Louis University Medical Center's Center for Vaccine Development. Jan Tennant, vaccine study coordinator, swabs Williams' arm.
As the mother of a 12-year-old daughter, Williams says she has been deeply affected by photos she's seen of infants born with microcephaly after their mothers were infected by the Zika virus during pregnancy. So when the Saint Louis University Medical Center's Center for Vaccine Development requested volunteers to participate in a double-blind, placebo-controlled study to test a new Zika vaccine, she jumped at the chance.
"I imagined what those babies' parents were going through, and it just touched me," she says. "I'm not afraid of lending myself to science, so I figured this was a good opportunity to help out the rest of the world."
Williams was one of 30 volunteers to participate in the phase 1 trial. (See below.) SLU is the only vaccine center conducting the trial, which is funded by the National Institutes of Health. The volunteers were all enrolled by mid-January and had received their requisite two inactivated vaccinations by mid-February. Study subjects will get blood draws for a year following their second injection so researchers can measure how immunity to the vaccine changes over time.
It will be two years before Williams learns whether she received the actual vaccine or just a saline solution, but she says she's happy just to know her participation may help. "I feel like the small risk I'm taking is worth it for the benefit it may provide others," she says.
Williams is just one of approximately 10,000 community volunteers who have been enrolled in more than 300 clinical trials at the university's vaccine center to help develop vaccines for infectious diseases such as influenza, smallpox, tuberculosis and dengue, a virus spread by the Aedes mosquito. Established in 1989 — and continuously funded since its inception by more than $158.5 million in contracts and grants from the NIH and others — the center is nationally recognized as a leader in research and development of vaccines to protect people against global pandemics.
Defending humankind
Dr. Daniel Hoft directs the center and the Saint Louis University School of Medicine's division of infectious diseases, allergy and immunology. He treats patients through the university's academic medical group, SLUCare, and is an infectious diseases expert at SSM Health Saint Louis University Hospital on the campus of the medical school. Hoft believes the vaccine work he and his colleagues are involved in is of critical importance.

Hoft
"If you look at some of the most recent epidemics — Ebola, H1N1, Avian flu, Zika — it's clear that no other global health problems can threaten massive death or serious illness as much as infectious diseases. And air travel has only added to the speed at which these epidemics spread," he says.
Vaccines, he says, are a triumph of modern medicine, and the development of new ones has the potential to impact the arc of human history.
"Most people don't realize that one of the top 10 leading causes of death in the U.S. is influenza," says Hoft. "Though we are still trying to develop a universal vaccine (for influenza), the vaccines we do possess have been successful in preventing disastrous outbreaks of flu. And while medicine has made progress in treating heart disease and cancer, no other field, besides infectious diseases, can claim that it has actually eradicated disease as we have done for smallpox."
Bioterrorism defense
From its earliest days until 2001, Hoft says, the center was known for its work on vaccines for hepatitis C and dengue, its research on the FluMist nasal spray influenza vaccine and its BCG (Bacillus Calmette-Guérin) vaccine for tuberculosis, which protects most children from the most severe complications of TB.
In 2001, after the 9/11 attacks on the World Trade Center and the Pentagon, the center's focus changed to biodefense. Although smallpox had already been eliminated through vaccination, for example, the U.S. government feared the virus could be used as a bioterror weapon. Research at the SLU center proved that existing, stockpiled Dryvax smallpox vaccine could be diluted to protect 10 times as many people. Stockpiles of anthrax and plague vaccines also were established, says Hoft.
Rarified status
With the opening of the state-of-the-art, $82 million Edward A. Doisy Research Center on the Saint Louis University medical campus in 2007, the center was able to establish biosafety level 3 labs — a classification that enables research with microbes that, if inhaled, can cause serious disease or death. The classification allowed it to qualify as a Vaccine and Treatment Evaluation Unit, or VTEU, and compete for NIH contracts to continue studying how to protect people from infectious diseases, including emerging threats. It also allowed the 75 center staff members — including 21 SLU faculty members — to collaborate more easily with immunologists, biochemists and microbiologists who also have offices at Doisy.
The center's VTEU status, says Hoft, enabled it to contribute to national preparedness in 2009, when a new pandemic, H1N1 (swine flu), swept the nation, and in 2013, when H7N9 (avian influenza) began infecting humans in China.
In 2013, the center also became one of an elite group of nine VTEUs — including Baylor College of Medicine in Houston; Duke University in Durham, N.C.; Vanderbilt University in Nashville, Tenn.; and Emory University in Atlanta — selected by the NIH to bid on nearly $1 billion in projects over the next 10 years.
But proposed cuts to the NIH budget under consideration in Washington, D.C., could severely limit available funding. Dr. Sarah George, the principal investigator for the Zika vaccine study at Saint Louis University, says with respect to the proposed budget cuts, "We are very concerned about how these severe budget cuts, if implemented, will impair vaccine development and research to multiple diseases, including Zika, Ebola, bird flu and many others. It cannot be overstated that these (proposed) cuts are extremely harmful to public health and safety."
The center hopes it will have the chance to, among other things, continue research on a new vaccine that uses T cells to induce immunity to tuberculosis — Hoft's particular area of interest. The VTEU status also has given staffers the opportunity to pursue "omics" research that uses genomic sequencing techniques to study the body's immune responses. Among them: transcriptomics, studying RNA molecules to tell when and where on DNA molecules genes are turned on and off in cells; proteomics, examining the structure and function of proteins like antibodies that are part of the immune system; lipidomics, studying lipids and their role in cellular signaling; and metabolomics, examining metabolites produced during digestion to see how end chemical reactions in one molecule influence beginning chemical reactions in another molecule.
Prevention is the best defense
Though Hoft says he is excited and proud to be "riding this wave of new technology," he says the center's mission has not changed.
"Ultimately, our focus is still on keeping people healthy," he says. "We know that vaccines are critically important to the goal of protecting the public."
And, he adds, that goal is shared by St. Louis' SSM Health, which, as part of its mission, sees every patient encounter as an opportunity to improve somebody's health.
"Today's health care systems have refocused their energies on providing preventative treatment to keep as many people as possible out of the hospital," says Hoft. "Of course, we still need to provide hospital care when necessary, but our health care dollars are better spent protecting people from disease than salvaging them once they contract disease."
|
Researcher says Zika/dengue vaccine may be ready for market by next year
Most Americans learned about the Zika virus — and its threat as a new global pandemic — in 2015, when Brazilian women mysteriously began giving birth to babies suffering from microcephaly, severe brain malformations, and other birth defects in unprecedented numbers.
But infectious disease physicians like Dr. Sarah George at Saint Louis University Medical Center's Center for Vaccine Development, who specializes in flaviviruses like yellow fever, dengue, West Nile and Japanese encephalitis — all spread by mosquitoes — were already well aware of Zika.
The virus was actually first isolated in a rhesus macaque monkey in the Zika Forest of Uganda in 1947, says George. From there, it was found throughout Africa and Southeast Asia, but with few cases in humans. Those that did occur resulted in relatively mild symptoms — headache, rash, fever, joint pain and conjunctivitis.
"The first real outbreak in humans was in 2007 on the island of Yap (part of the Federated States of Micronesia)," she says. "The next sizable outbreak was in French Polynesia and Tahiti in 2013-14. Then, of course, it arrived in Latin America and the Caribbean by 2014-15, and was determined to be transmissible through sexual relations in addition to the Aedes mosquito. And by 2016, it was discovered in South Florida and Texas."
That's when the National Institutes of Health put out a request to its elite group of nine Vaccine and Treatment Evaluation Units, including SLU's center, for proposals to develop protocols to test an investigational vaccine. SLU was chosen in November to conduct the first early stage clinical trial of a Zika vaccine. George says there are least five different vaccine candidates, maybe more. Many of the vaccines are being developed by industry.
Normally, bringing a new vaccine to market can take up to 20 years. But the potential for a public health emergency (in this case, due to the severe consequences for babies infected in the womb), plus the existence of a licensed
vaccine developed for Japanese encephalitis, is fast-forwarding the process.
"The vaccine candidate, ZPIV (Zika Purified Inactivated Virus), is modeled after the Japanese encephalitis vaccine and is being developed by Walter Reed Army Institute of Research," says George, who is the principal investigator at SLU. Parallel studies are being conducted by Walter Reed, Beth Israel Deaconess Medical Center in Boston and the National Institute of Allergy and Infectious Diseases' Vaccine Research Center.
Another study is underway at Ponce Health Sciences University in Puerto Rico to examine the vaccine's safety in participants who already have been naturally exposed to dengue virus, the Zika virus, or both. George says any vaccine that is licensed would ultimately be used in people who have exposure to dengue and/or Zika.
A phase 2 pharmaceutical study already is planned for the fall, and a phase 3 study, potentially involving 30,000 people in half a dozen countries where the viruses are endemic, would follow before full licensure by the Food and Drug Administration could occur.
"We are hoping to have a product available for emergency use if needed in 2018," says George. "Ultimately, we would like to have a vaccine to immunize men and women before they reach childbearing years."
George says the importance of "getting ahead of the game" when it comes to infectious diseases like Zika or Ebola cannot be overestimated.
"At the Center for Vaccine Development, we consider this to be extremely important work, not only scientifically, but also because of Saint Louis University's mission as a Jesuit institution to relieve and prevent suffering in the world," she says.
— RENEE STOVSKY
|
Copyright © 2017 by the Catholic Health Association
of the United States
For reprint permission, contact Betty Crosby or call (314) 253-3490.