BY: JEFFREY G. SHAW
Jeffrey Shaw is genetics counselor, Cancer Center, Penrose-St. Francis Health
Services, Colorado Springs, CO.
The First Article in a Series about the Significance of Genetic Science
for Catholic Health Care
Genomics and Our Ministry
Advances in Genomics have already begun to have an impact on health care
delivery. The opportunities these advances create for more effective diagnosis,
prevention, and treatment of many diseases offer considerable promise for reducing
the suffering caused by disease and disability. For this reason, it is essential
that Catholic health care attend to these developments and integrate them into
its own delivery of care when and where appropriate, but not without carefully
considering the ethical dimensions of these developments from the perspective
of the Catholic theological and ethical tradition.
With this issue of Health Progress, a group of scientists, clinicians, and
ethicists will examine some of the ways genomic advances are affecting health
care today. Our introductory article is by Jeff Shaw, a genetics counselor at
the Cancer Center, Penrose–St. Francis Health Services Cancer Center, Colorado
Springs, CO.
Subsequent issues will feature closer examinations of how genomic advances
are influencing specific areas of medicine, such as perinatology/pediatrics,
oncology, and cardiology. These closer examinations, written by experienced
practitioners, will be accompanied by commentary from health care ethicists,
who will provide insight into some of the related ethical implications that
need to be considered.
—Scott McConnaha
The years surrounding the turn of the 21st century mark the entrance of genetics
into mainstream medicine. Thanks to the hard work and dedication of scientists
(and to powerful mainframe and desktop computers), the Human Genome Project
(HGP) achieved what had been thought to be impossible a few decades earlier.
The HGP completed the mapping of the letters of genetic code needed to build
a human being.
We humans have 3.2 billion letters of code in our DNA (deoxyribonucleic acid),
making up an estimated 20,000 to 30,000 genes. Our ability to understand this
very basic blueprint will transform the practice of medicine. Indeed, the transformation
has already begun. Ultimately, all medical practitioners will need to understand
the world of genetics, as will consumers of health care. In this article, I
hope to give the reader a basic introduction to genetics. I will describe how
genetics is involved in medical care today and make some guesses about its impact
on the future.
DNA, Genes, and Things
We hear almost routinely now about how a gene has been identified as being
involved in some medical condition or trait. Hardly a day passes without the
announcement of another gene being identified in those 3.2 billion letters of
genetic code. DNA is the molecule of life. All the information necessary to
create a human is encoded in it. The human blueprint encoded in our DNA makes
tens of thousands of different proteins. These proteins make up all the tissue
and molecules of the body.
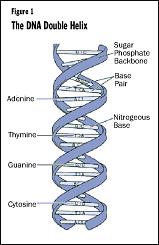
The achievement of the HGP rests upon the work of two men, James Watson and
Francis Crick, who in 1953 discovered the "double helix" structure
of DNA. This double helix looks something like a twisted ladder (see Figure
1).The long sides of the ladder are a repeating sugar-phosphate-sugar-phosphate
polymer. The rungs of the ladder are formed by "base" pairs. The four
"bases" are adenine (A), guanine (G), cytosine (C), and thymine (T).
Each side of the helix is complementary to the other—that is, A will always
match up to T, and C will always match up to G.
The
structure of DNA makes possible:
- The storage of vast amounts of information
An easy method of replicating itself: to copy, each side of the double helix
will separate, unwind, and copy itself.
- Protection against information loss from damage to the DNA
Only one side of the helix is used as the actual blueprint. The other side is
used for structure and as a means of copying DNA into a new strand. A gene is
a specific length of DNA that makes a protein product. The purpose of genes is
to provide the blueprint from which is made all the proteins that constitute our
bodies.
Let's Make a Protein
You can picture a gene as a sentence written in a language that only has
four letters in its alphabet. For example, the coding side of a DNA strand could
be: ATGCAGCAGTTATTTCCGTAA. In genetics, every "word" in a sentence
is three letters long. The genetic "sentence" we have just written
would thus read:
ATG CAG CAG TTA TTT CCG TAA.
Now, the DNA code is transcribed into a different molecule, called messenger
RNA (mRNA). There are three important differences between DNA and mRNA:
- RNA (ribonucleic acid) is single stranded, not double.
- The sugar is ribose, not deoxyribose.
- The base Thymine (T) is replaced by the base Uracil (U).
So, after transcription, our "sentence" reads: AUG CAG CAG UUA UUU
CCG UAA. It is from this mRNA code that our final protein will be made.
Next, we will translate our code into our protein. Every three-letter "word"
is part of a set code appropriately referred to as "the genetic code" (see Figure
2). Every three-letter "word" is a code for one of 20 specific amino acids
used to make protein. As you can see from the table, the genetic code is redundant.
For example, CUU, CUC, CUA, and CUG all code for the amino acid Leucine. AUG,
which is always found at the beginning of a gene, is the code to signify where
to start reading a gene. You will also note that UAA, UAG, and UGA do not code
for an amino acid. They will exist only at the end of a gene, to tell us to
stop building.
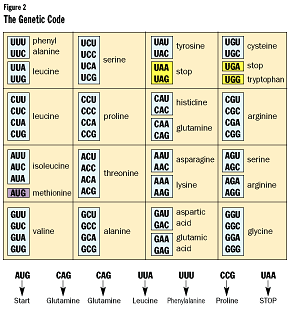
These amino acids are strung together to make our protein: glutaime-glutaime-leucine-phenylalanine-proline.
This protein might be a part of a muscle cell, an eye pigment, or some other
part of the body. The gene we created above is very small. The average gene
has 3,000 letters of code. The largest discovered so far is the dystrophin gene,
which has 2.4 million letters of code. If a letter of code of a gene is changed,
this is called a mutation, which can lead to disease. A mutation could
involve simply changing one letter of code to a different letter, or could refer
to deleting, adding, or rearranging single or multiple letters of the code of
a gene.
It is important to note that every human being has the same 20,000 to 30,000
genes (unless he or she has an inherited condition or a problem with chromosomes).
It is variations in these genes that give each of us our individual physical
appearance and predispose us to certain diseases. In my practice, I work with
people at risk for inherited cancer predispositions. I often hear a patient
say, "My sister has the gene for breast cancer, and I want to be
tested to see if I have it." By putting it this way, the patient makes
it sound as if her sister had a unique gene causing breast cancer. In fact,
the sister has the same genes as everyone else. We all have the genes that control
the growth of our breast tissue. What the patient is trying to say is that her
sister has a mutation in one of these genes, that the mutation prevents the
gene from working correctly, and that this problem gives her an increased risk
for breast cancer.
Chromosomes: The Big Picture
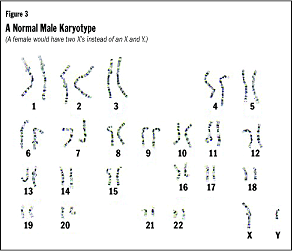
Chromosomes are the structures that "hold" our genes. The estimated 20,000
to 30,000 genes that make a human being are located on 46 chromosomes. Each
chromosome is simply a long, tightly coiled strand of DNA. These 46 chromosomes
occur as 23 pairs. We get one of each pair from our mothers (in the egg) and
one from our fathers (in the sperm). The first 22 pairs of chromosomes are labeled
from 1 to 22, from the longest to the shortest. We might note here that, when
it was first viewed under a microscope, the 21st chromosome looked longer than
the 22nd. Improved technology revealed that the 21st chromosome was actually
shorter than the 22nd. The 21st chromosome has 46 million letters of code, whereas
the 22nd has 49 million letters. The longest chromosome, No. 1, is a DNA strand
with about 246 million letters of code. The last pair are the sex chromosomes,
labeled X and Y. Females have two X chromosomes (XX), whereas males have an
X and a Y chromosome (XY) (see Figure 3).
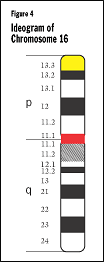
Each chromosome has a p arm (the long arm) and a q arm (the short one). Some
chromosomes—13, 14, and 15, for example—have very small p arms. When
a karyotype is made (see below), the q arm is always put on the bottom and the
p on the top. The arms are separated by a region known as the "centromere"
(shown as red in Figure 4), which is the pinched area
of the chromosome.
Incidentally, chromosomes must be stained to be seen under a microscope. When
stained, they look like strings with light and dark bands.
What is a Karyotype?
A karyotype is an actual photograph of the chromosomes from one cell.
The cells analyzed are usually white blood cells taken from a patient or a prenatal
specimen. Once they have been stained, the chromosomes appear as banded strings
magnified a thousand times. The chromosomes can be seen only at a specific time—during
cell division, when they are tightly coiled up into the little packages we see
as chromosomes. They are analyzed by specially trained cytogenetic technologists,
cytogeneticists, or medical geneticists. Cytogenetics is the word for
the study of chromosomes. When seen in a karyotype, the chromosomes can appear
bent or twisted. This is normal and simply reflects the way they sit on a slide.
Chromosome Abnormalities
If a chromosome or a piece of a chromosome is either missing or duplicated,
the organism will also have missing or extra genes. A person who has missing
or extra information (genes) may develop problems in his or her health or development.
Chromosome abnormalities are usually sporadic occurrences. However, it is possible
to inherit a chromosome abnormality from a parent. Clinically relevant chromosome
abnormalities occur in approximately one percent of live births. There are hundreds
of specific chromosome abnormalities that could occur in an individual. However,
having an entire extra or missing chromosome is usually incompatible with life.
About half of all conceptions end in pregnancy loss, and it appears that 25
percent of such losses are due to abnormalities in the number of chromosomes
in the embryo. Therefore, the bulk of chromosome abnormalities have their impact
in the prenatal period, leading to miscarriage.
Probably the best-known chromosome abnormality is called Down syndrome. People
with Down syndrome have three copies of the 21st chromosome instead of two.
Usually, this is due to an accident that has occurred when a parent's egg
or sperm is made. Instead of getting one 21st chromosome from each parent, the
person gets one from one parent and two from the other. Possessing an extra
21st chromosome, the person has three copies of the genes on this chromosome
instead of two. This leads to recognizable birth defects, mental retardation,
and other health problems.
Inherited Single-Gene Disorders
Inherited single-gene disorders are the result of mutations in single-mutated
genes and generally have a very large impact on the health of the person involved.
Literally thousands of diseases are caused by mutations of a single gene. The
most complete listing of known single-gene disorders can be found at Online
Mendelian Inheritance in Man (OMIM, which can be found at www.ncbi.nih.gov/entrez/query.fcgi?db=OMIM).
OMIM is an enormous online database that catalogs human genes and genetic disorders.
In 1865, Gregor Mendel discovered how inherited diseases are passed from generation
to generation. Although it now appears that Mendel misrepresented his data,
he did discover the basic fundamentals of inherited single-gene disorders. The
modes of inheritance of these single-gene disorders are referred to as "Mendelian
inheritance," in his honor. These modes of inheritance are described as
recessive, dominant, and X-linked. The words "dominant" and "recessive"
refer to a trait or condition, not a gene.
Recessive Conditions
In the case of either dominant or recessive conditions, we need to understand
how many genes of a pair must be damaged in order to cause disease.
Homozygous Wild Type No mutation exists in either gene. Both genes (one
from the father and one from the mother) are working correctly. A person of
this type would not be affected by a recessively inherited condition because
the genes are working as they should. Since both genes are working, the person
possessing them can pass only a working gene down to his or her children. The
genetic nomenclature used to show that both genes are working correctly would
be w+/w+.
Heterozygous A mutation exists in only one of the genes. Therefore,
one of the genes is working correctly and the other is not. This would be listed
as w+/w-. In recessively inherited conditions, if one gene is working and one
is not, the person involved is considered a "carrier." Because one
gene is working correctly, the person will not develop the disease. If that
person becomes a parent, he or she will have a 50 percent chance of passing
on the faulty gene to the child.
Homozygous Mutation Neither gene is working, due to a mutation in the
genetic code of both genes. The person would be listed as w-/w-. Since neither
copy of the gene is working correctly, the person would be affected with the
recessive condition.
Let's say that a man and a woman have children. Both parents are carriers
of the same faulty gene, a predisposition for sickle cell disease, for example.
For each of the woman's pregnancies, there would be:
- A 25 percent chance that the child is neither a carrier of the disease nor
affected by it (w+/w+)
- A 50 percent chance that the child is a carrier but not affected (w+/w-)
- A 25 percent chance that child is affected (w-/w-)
Concerning recessive inheritance, the key points to keep in mind are the following:
- Both parents need to be carriers of the same faulty gene.
- Because a child of such parents must, to develop the condition, inherit
faulty genes from both parents, he or she is unlikely to have a family
history of it.
- For each of the woman's pregnancies, there is a 75 percent chance that
the child will not be affected.
Sickle cell disease is a recessively inherited condition most common in people
of African descent. The alteration of just one letter of genetic code in the HBB
gene (a GAG changed to GTG) will cause the disease. The change in this one letter
will cause the red blood cells to collapse. Sickled red blood cells plug microcirculation,
resulting in severe bone pain; damage to internal organs, especially the heart,
lungs, and kidneys; increased risk for cerebrovascular accidents; infection; heart
attacks; and chronic anemia.
Dominant Conditions
Two possibilities are found in dominantly inherited conditions.
Homozygous Wild Type Both genes are working correctly. A person of this
type would not be able to pass a faulty gene down to his or her children and
would not be affected by the condition. The person would be listed as w+/w+.
Heterozygous One gene is working correctly and the other is not. The
person would be listed as w+/w-. The difference between dominant and recessive
inheritance is that, in dominantly inherited conditions, both genes need
to be working correctly in order to preclude the disease.
The key points to keep in mind about dominant inheritance are the following:
- Only one parent needs to have the faulty gene.
- The faulty gene is equally likely to come from a male or female parent.
- An individual needs to inherit only one faulty gene in order to be affected
by the condition.
- A family history is likely to exist, showing the condition coming down through
one side of the family.
- For each pregnancy, there is a 50 percent chance that the offspring will
be affected and a 50 percent chance that he or she will not be affected.
Some dominant conditions have
reduced penetrance, which means that not
everyone who has the faulty gene will develop the condition. In some cases of
reduced penetrance, the condition may "skip" generations. Inherited
conditions can also have
variable expressivity: Some people may have a
very mild form of the condition, whereas others are affected very severely. Reduced
penetrance and variable expressivity can make it difficult for a geneticist to
interpret a family history.
There are thousands of dominantly inherited traits and diseases, including
Huntington's disease and many inherited cancer predispositions.
X-Linked Inheritance
In a condition said to be "X-linked," the faulty gene is located
on the X chromosome. Women have two X chromosomes and men have one X and one
Y chromosome. A man inherits his Y chromosome from his father and his X chromosome
from his mother. For most X-linked conditions, women are carriers and not affected,
since they have one X chromosome that is working correctly. If a man's
one X chromosome carries a faulty gene, he will be affected because,
unlike women, he has no other.
Key points to be kept in mind about dominant inheritance are:
- Women are carriers and usually not affected by the condition.
- If a woman who carries the faulty gene gives birth to a boy, there is a
50 percent chance he will inherit the faulty gene and have the condition.
- If a woman who carries the faulty gene gives birth to a girl, there is a
50 percent chance she will be a carrier.
- If a male carries the faulty gene, every one of his daughters will be a
carrier (because he only has one X chromosome to pass down). None of his sons
will be either affected or carriers (because he passes down a Y chromosome
to his sons).
Fragile X-syndrome, color blindness, Duchenne muscular dystrophy, and hemophilia
are X-linked conditions.
Polygenic or Multifactorial Disease
Polygenic or multifactorial diseases are complex conditions that result
from the interaction of suboptimal genes inherited from one's parents and
one's environment and lifestyle. Poor vision, rheumatoid arthritis, diabetes,
heart disease, hypertension, depression, addiction, and cancer are multifactorial
conditions.
Here's an example of the way interaction between suboptimal genes and
lifestyle works: I will inherit genes from my parents that are involved in making
the proteins that break down cholesterol in the food I eat. However, these genes
may have variations that cause them to work less well than they should. Now
suppose that I live my life on a high-cholesterol diet. Because of those inherited
gene variations and the high-cholesterol diet, I will have a greater chance
of developing heart disease than a person who has a healthy diet and does not
have my gene variations.
Acquired "Somatic" Conditions
In contrast to chromosomal, single-gene, and multifactorial conditions,
acquired or "somatic" genetic disorders are not due to damaged genes
in the egg or sperm, or inherited genes. Instead, they result from damage done
to genes by aging. Cancer is the paradigm of acquired genetic damage.
Cancer is developed through mutations in the genes that control the growth
of a cell. Tumor-suppressor genes make proteins that are either directly or
indirectly responsible for controlling the growth of a cell. Should a carcinogen—tobacco
use, for example—damage these genes, the cell loses control of its growth
and may become a malignancy. All cancers are "genetic" diseases because
they are caused by damaged genes. This does not mean that all cancers are "inherited."
The mutations that damage the genes in question have been acquired during the
person's lifetime, not passed down from parents. In fact, cancers that
result from an inherited predisposition are much less common than those due
to acquired mutations.
As in other medical fields, genetic medicine has its exceptions to the rule
and its new discoveries. Over the past 10 to 20 years, we have discovered many
intricacies in the way diseases can be inherited, including mitochondrial inheritance,
"anticipation," uniparental disomy, and imprinting. Information about
these intricacies can be found at the Mountain States Genetics Network, www.mostgene.org.
Current Uses of Genetics in Medicine
Because there is a seemingly unending discovery of genes that when damaged
can cause disease, the number of clinically available genetic tests will continue
to increase as well. At present, many of these tests are for very rare inherited
diseases. The next decade will see a significant increase in the number of tests
available for common complex genetic diseases—diabetes, heart disease,
and arthritis, for example.
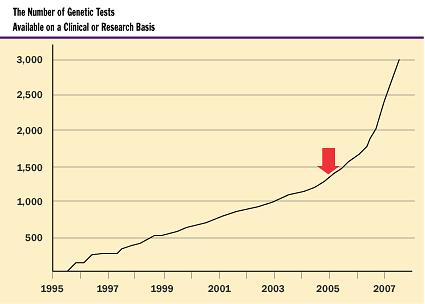
How is genetics likely to affect medicine in the future? Here are some examples.
Diagnosis Genetic analysis can now break down the classification of
some conditions—such as colon cancer and breast cancer—into finer
categories. This is important because an ability to classify diseases more precisely
will suggest more appropriate treatments. As the genetic underpinnings for disease
become more completely understood, this refinement will also become possible
for heart disease, schizophrenia, and many other medical conditions.
Pharmacogenomics This is the tailoring of drugs to particular patients,
whose responses can be predicted by genetic "fingerprinting." For
example, cancer patients facing chemotherapy may experience fewer side effects
and improve their prognoses if they first get a genetic "fingerprint"
of their tumors. The "fingerprint" will reveal which chemotherapy
choices are most likely to be effective. In general, the better understanding
of genetics can be said to promise a future of precise, customized medical treatments.
Prognosis Diagnosing ailments more precisely will result in more reliable
predictions about the course of a disease. For example, a genetic workup can
inform a patient with high cholesterol levels how damaging that condition is
likely to be. Doctors treating prostate cancer will be able to more accurately
predict how aggressive the tumor will be. For many diseases, such genetic information
will help patients and doctors weigh the risks and benefits of available treatments.
Prevention (presymptomatic predisposition profiling) Once scientists
can determine which DNA sequence changes in a gene can cause disease, healthy
people can be tested to see whether they risk developing conditions such as
heart disease, diabetes, or prostate cancer later in life. An advance warning
can be a cue to start a vigilant screening program, to take preventive medicines,
or to make diet and lifestyle changes that might preclude the disease altogether.
Gene Therapy This involves replacing a damaged gene. For more than a
decade now, patients in small groups have undergone gene therapy in clinical
trials. But this remains an experimental treatment. Eventually, gene therapy
will likely become a common treatment for many single gene conditions.
Gene-Based Pharmaceuticals Great medical benefit will likely occur from
drug design that is guided by an understanding of how genes work and of the
exact process through which disease occurs at the molecular level. Pharmaceutical
researchers will then begin work with a clearer notion of the kind of molecule
they will need to treat specific diseases, rather than relying on chance and
having to screen thousands of molecules to find an effective drug. Because rationally
designed drugs will act in very specific ways, they are less likely to have
significant, even damaging, side effects. In the next 10 to 25 years, new molecular
drugs, protein replacement therapies, and designer molecules created to block
disease causing proteins will become commonplace in the pharmaceutical industry.
Genetic discoveries are having a great impact on contemporary health care.
Medical genetics covers the entire human lifespan, from preconception to prenatal
and from pediatric medicine to adult medicine. Genetics is also involved in
diseases of every organ system. Those of us who work with patient care will
need to take steps to ensure that medical genetics is intelligently incorporated
in the way we go about practicing medicine.
DNA Tidbits
Here are some little-known facts about DNA:
- Less than 2 percent of the 3.2 billion letters of code makes a protein.
- You could fit 5 million strands of DNA through the eye of a needle.
- If you were to pull all the chromosomes into long strands of DNA, there
would be six to seven feet of DNA in each cell.
- If all of the DNA in an average-sized adult were placed end to end, it would
circle the globe 114,000,000 times.
- 99.9 percent of the letters of code are exactly the same in all human beings.
Copyright © 2005 by the Catholic Health Association of the United States
For reprint permission, contact Betty Crosby or call (314) 253-3477.